Abstract
Introduction: Interactions between tumor and the TME occur through direct cell contacts and secreted factors such as cytokines and growth factors may influence behavior of tumor cells and response to therapy. Development of clinically applicable tumor models for screening therapeutics is desired to enhance our understanding of these mechanisms. We harnessed a novel MF-SC technology and an innovative 3D biomimetic tumor model to improve our understanding of the role of TME with immunotherapy and NHL tumor biology.
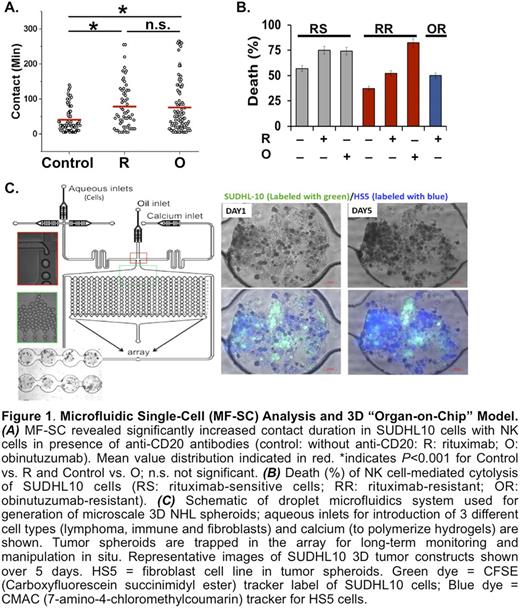
Methods: Rituximab (R)- and obinutuzumab (O)-resistant (RR & OR) B-NHL cells (SUDHL4, SUDHL10 & Raji) were developed for evaluation of anti-CD20 resistance. Patient derived cell lines were obtained from newly diagnosed DLBCL patients. Functional response of anti-CD20 resistant & susceptible B-NHL cells were determined at SC levels in a customized MF droplet array platform. Single CD16+ NK cells were co-encapsulated with B-NHL cells at 1:1 effector-target (E:T) ratio in picoliter-volume droplets in presence/absence of R & O. Dynamic cellular interaction between E-T was monitored via live-cell time-lapse microscopy over 4-6 hrs to assess interaction profiles (eg, contact duration, frequency, and single vs serial interaction) and functional cytotoxicity. Target cell kill kinetics was assessed by loss of Calcein AM & ethidium homodimer uptake. Further, we used systems biology analyses (ie, Ingenuity Pathway Analysis (IPA) & Gene Set Enrichment Analysis (GSEA)) to identify associated biologic pathways. Additionally, the MF-SC platform was used to develop microscale 3D multicellular constructs to monitor effect of cancer-stroma interaction in a simulated TME. Heterogeneous tumor spheroids were generated using composite alginate hydrogels and varying combinations of NHL cells, fibroblasts & immune cells. Effect of TME (ie, cell composition and biological activity of immune cells) in the presence/absence of lenalidomide was evaluated based on extent of target cell survival/death (differentially labeled using CellTrace cell labeling & tracking dyes).
Results: Development of anti-CD20 resistance in B-NHL cells resulted in decreased expression of CD20, loss of R binding, and decreased cytotoxic activity. Using IPA and GSEA systems biology analyses of RR and OR B-NHL cells, we observed conserved down-regulation of IFN-α/γ, TNF-α, inflammatory response, hypoxia and complement response pathways, and upregulation of MYC targets, which predicted overall immune suppression in all CD20 resistant cells. With MF-SC, we determined that NK cells induced variable duration of contacts with SUDHL10 cells irrespective of anti-CD20 resistance. While the single or multiple contacts made between NK & target cells occurred for an average of 45 minutes, addition of R or O resulted in significantly extended contact time (77 & 79 minutes; P <0.001 each) (Fig 1A). These contacts resulted in cell death with NK cells alone in 56% R-sensitive cells, which increased to 75% with addition of R (P <0.01). In RR cells, R increased NK cell-mediated target death from 37% (NK cells alone) to 52% in the presence of rituximab, but with perturbed cell-to-cell contact. The addition of O increased cell death (83%) in RR SUDHL10 cells (P <0.005) (Fig 1B). Primary DLBCL cells were also tested in this platform; there was NK-mediated cytotoxicity/death seen (68% in 80 minutes). We also developed a novel MF 3D lymphoma spheroid model using composite hydrogels optimized to host B-NHL cells (Fig 1C). In the presence of activated immune and stromal components, lenalidomide induced potent 2.5 fold increase in SUDHL10 cell death vs 3D cultures that lacked activated immune or stromal cells, which recapitulated the physiological mechanisms of TME-dependent lenalidomide drug activity.
Conclusions: We identified that resistance to R & O occurs through biologically conserved mechanisms. Results in MF-SCs revealed that interactions between NHL tumor and immune cells is a highly dynamic process occurring through multiple cell contacts and that presence of R & O shift these kinetics; this was an important factor in determining cytotoxic immune activity. In addition, experiments based on reconstituted 3D NHL tumor spheroids validated the influence of TME mediating the activity of immunomodulatory drugs. This innovative MF-based 3D NHL spheroid model may be further leveraged for the discovery of immune-based therapeutic applications.
Evens: Celgene: Consultancy; Merck: Consultancy; • Spectrum Pharmaceuticals: Consultancy; Novartis: Consultancy; AbbVie: Consultancy; Seattle Genetics: Consultancy; Pharmacyclics: Consultancy; Kite Pharma: Consultancy; Millennium: Consultancy; Affimed: Consultancy; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal